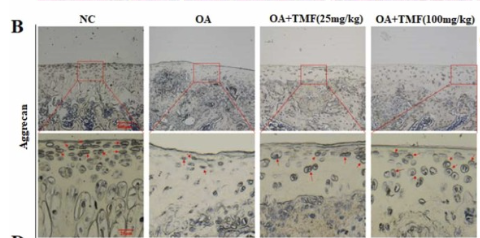
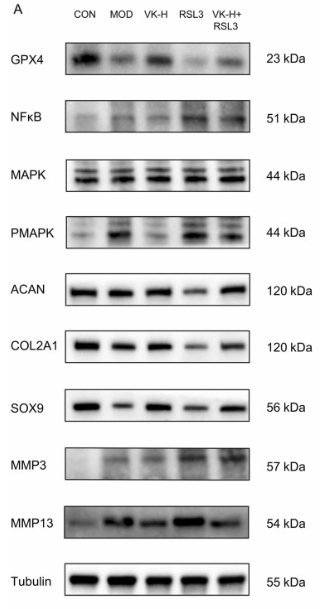
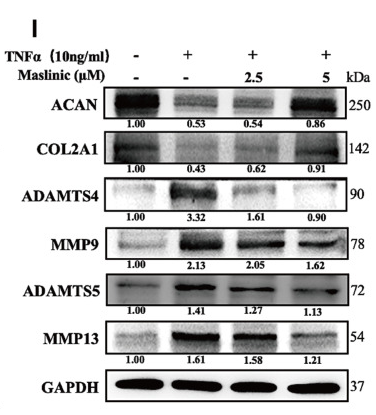
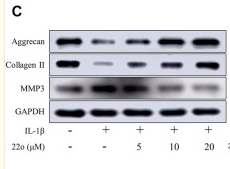
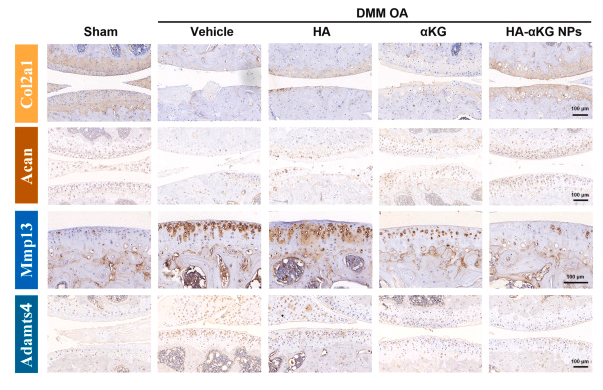
ab17521 Aggrecan Antibody
品牌 |
|
|---|---|
产品货号 |
|
来源种属 |
Rabbit |
抗体克隆 |
Polyclonal |
来源亚型 |
IgG |
实验方法 |
WB,IHC,IF,ICC |
实验种属 |
Human,Mouse,Rat,Rabbit,Pig,Dog,Chicken,Bovine,Horse,Sheep |
偶联标记 |
Unconjugated |
目的蛋白 |
Aggrecan |
产品规格 |
50μl,100μl,200μl |
产品报价 |
¥1500/¥2750/¥3600 |
实验应用
Western blotting
Recommended dilution: 1:1000-3000
Immunofluorescence
Recommended dilution: 1:100-1:500
immunocytochemistry
Recommended dilution: 1:100-1:500
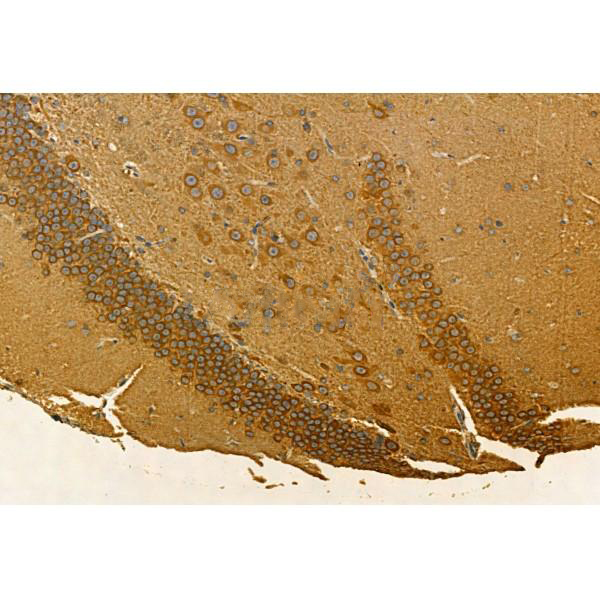
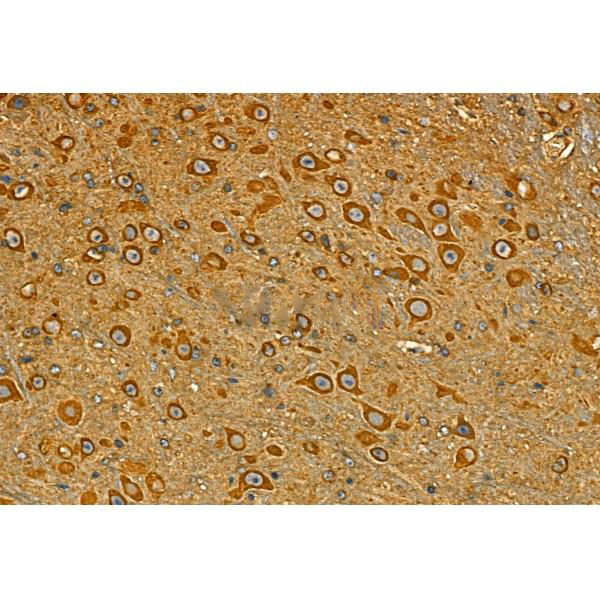
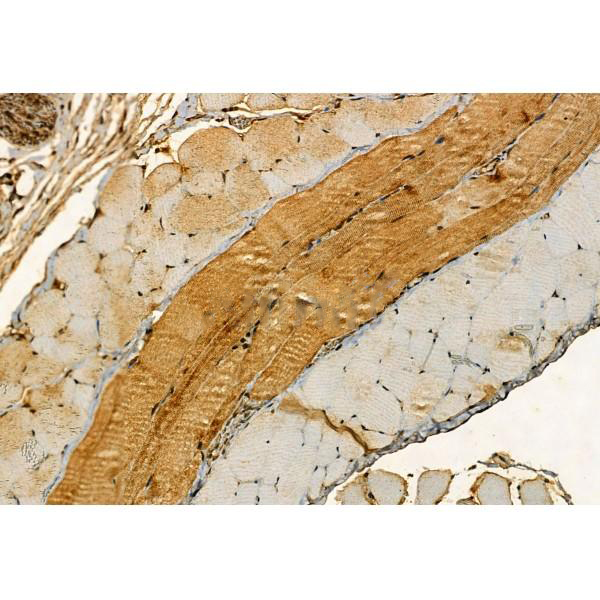
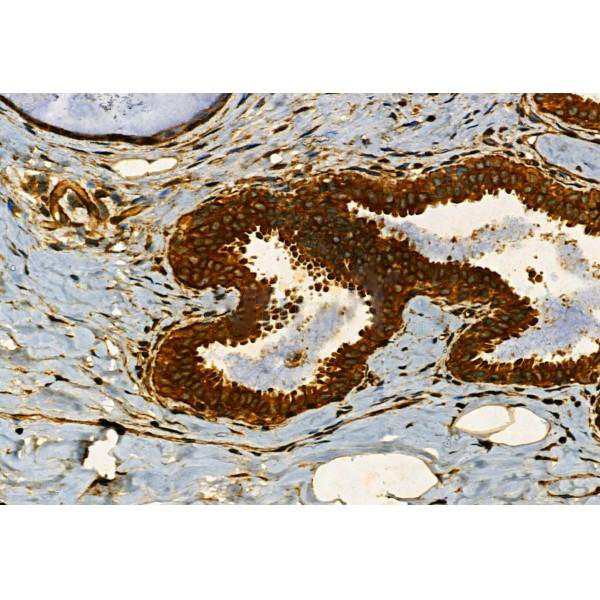
Immunohistochemistry
最佳稀释倍数与浓度应由实验研究人员确认
产品说明
产品背景
This proteoglycan is a major component of extracellular matrix of cartilagenous tissues. A major function of this protein is to resist compression in cartilage. It binds avidly to hyaluronic acid via an N-terminal globular region.Description
Rabbit polyclonal antibody to Aggrecan
Applications
WB, IF, ICC, IHC.
Immunogen
Aggrecan Antibody detects endogenous levels of total Aggrecan.
Reactivity
Human, Mouse, Rat.
可预测:Pig(100%), Bovine(%), Sheep(%), Rabbit(%), Dog(%)
Molecular weight
70,150,250 kDa; 261kD(Calculated).
Host species
Rabbit
Ig class
Immunogen-specific rabbit IgG
Purification
Antigen affinity purification
Full name
Aggrecan
Synonyms
ACAN; AGC 1; AGC1; AGCAN; Aggrecan 1 (chondroitin sulfate proteoglycan 1, large aggregating proteoglycan, antigen identified by monoclonal antibody A0122); Aggrecan 1; Aggrecan core protein; Aggrecan proteoglycan; Aggrecan structural proteoglycan of cartilage; Aggrecan1; ATEGQV; Cartilage specific proteoglycan core protein; Chondroitin sulfate proteoglycan 1; Chondroitin sulfate proteoglycan 1 large aggregating proteoglycan antigen identified by monoclonal antibody A0122; Chondroitin sulfate proteoglycan core protein 1; CSPG 1; CSPG1; CSPGCP; JSCATE; Large aggregating proteoglycan; mcspg; mgsk16; MSK 16; MSK16; SEDK;
Storage
Rabbit IgG in phosphate buffered saline , pH 7.4, 150mM NaCl, 0.02% sodium azide and 50% glycerol. Store at -20 °C. Stable for 12 months from date of receipt.
Swissprot
P16112
产品图片
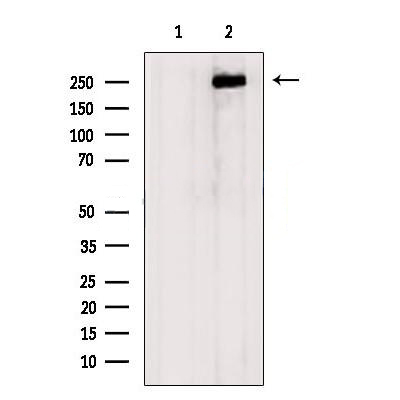
|
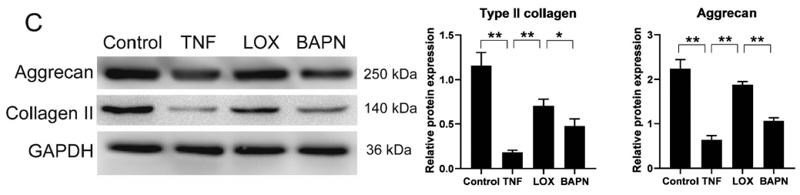
Western blot analysis of extracts from 3T3, using Aggrecan Antibody. The lane on the left was treated with blocking peptide. |